Introduction
Mosunetuzumab (mosun) is a CD20xCD3 bispecific antibody that has been FDA-approved for relapsed/refractory follicular lymphoma after two prior lines of therapy. Updated follow up of the pivotal study in relapsed/refractory patents (Sehn et al ICML 2023, median follow up 28.3 months) showed a CR rate of 60%. Among the patients in CR, 77% remained alive and progression free at 2 years. We hypothesized that mosun would be even more active in patients (pts) without prior lymphotoxic therapy. Therefore, we designed a response adapted study for pts with untreated follicular lymphoma (FL) and marginal zone lymphoma (MZL).
Methods
This is a single center, open label, investigator-initiated response-adapted clinical trial in untreated pts with FL or MZL with indication for treatment. Pts received subcutaneous mosunetuzumab monotherapy for 8 cycles (21 day cycle, Part A) employing step up dosing in cycle 1 (5 mg, 45 mg, 45 mg). Dexamethasone prophylaxis was administered through C2D1, and could be omitted after that for patients without CRS in the previous cycle. A PET/CT was performed after 8 cycles, and pts in CR were observed without further treatment. Pts with SD or PR could receive 6 cycles (21 day cycle, Part B) of polatuzumab vedotin and obinutuzumab, followed by an end of treatment (EOT) PET/CT. Total planned accrual is 42 pts. The primary endpoint is complete response (CR) rate. A pre-planned interim efficacy analysis is being reported after 20 enrolled pts. If 14 or fewer of the first 20 subjects do not achieve an objective response, the study will be suspended to enrollment. Additional planned safety analyses are in place with suspension rules to detect excessive G4 non-heme toxicity and G2+ CRS.
Results
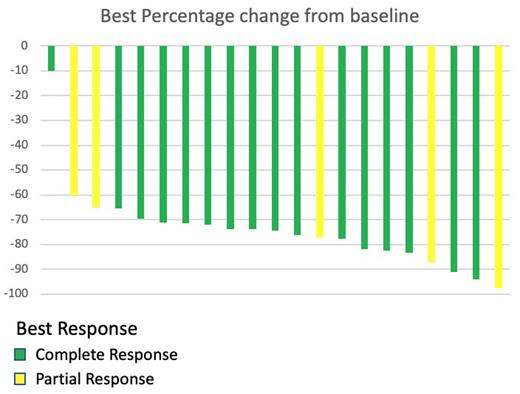
26 pts enrolled on study between March 24, 2022, and March 11, 2023, with 26 pts evaluable for safety, and 21 pts are evaluable for efficacy. Median age was 64 (42-83), including 16 (62%) age 60+. Histologies enrolled include G1-2 follicular lymphoma (19, 73%), G3A follicular lymphoma (4, 15%), and marginal zone lymphoma (3, 12%). The most common indication for treatment was symptomatic disease (19, 73%). All treatment was delivered as an outpatient. Three pts experienced a serious adverse event (one pt with G3 lung infection followed by G3 shingles, one pt observed overnight with G1 cytokine release syndrome, and one pt with a G2 URI). Other G3 non-lab AEs observed were diarrhea (1, 4%), syncope (1, 4%), and hypertension (1, 4%). Sixteen (62%) pts experienced G1 CRS, with no G2 or higher CRS observed or any grade ICANS observed. No pts required tocilizumab. All CRS events first occurred during cycle 1. Headache was common but low grade (G1 14, 54%, G2 1, 4%). Heme toxicity > G1 was uncommon, with G4 neutropenia (1, 4%), and G2+ thrombocytopenia (2, 8%). No G4 non-heme toxicity was observed. Nearly all pts (92%) experienced at least one injection site reaction, which were all G1 except a single pt with a G2 reaction. Seven (26%) pts experienced a treatment delay due to toxicity, primarily due to infection in 4 pts. Only one patient did not receive all doses of mosun - G3 ALT increase and prolonged G1 CRS led to discontinuation after cycle 1 (patient achieved CR). Twenty-one patients are efficacy evaluable, with an overall response rate (ORR) of 100%. Eighteen patients have completed mosun therapy, with an ORR and CR rate of 100% and 83%, respectively. Three patients proceeded to obinutuzumab and polatuzumab combination therapy, of whom two have completed all treatment (both converted to CR). All pts remain alive and progression-free. Updated data will be presented at the meeting.
Conclusion
Fixed duration mosun monotherapy achieves a high CR rate in untreated pts with follicular and marginal zone lymphoma with no G2+ CRS and no ICANS of any grade. Injection site reactions and headaches were common but reversible. Safety and efficacy has remained well within study suspension thresholds and accrual is ongoing to a goal of 42 total patients.
Disclosures
Lynch:Rapt: Research Funding; Seagen Inc.: Research Funding; TG Therapeutics: Research Funding; Cancer Study Group: Consultancy; Cyteir: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Bayer: Research Funding; Merck: Research Funding; SeaGen: Consultancy; Foresight Diagnostics: Consultancy; Abbvie: Consultancy. Poh:Incyte: Research Funding; Seattle Genetics: Consultancy; BeiGene: Consultancy; Acrotech: Consultancy. Shadman:AbbVie: Consultancy, Research Funding; MEI Pharma: Consultancy; Genmab: Consultancy, Research Funding; Janssen: Consultancy; Eli Lilly: Consultancy; TG Therapeutics: Research Funding; Kite, a Gilead Company: Consultancy; Fate Therapeutics: Consultancy; Vincerx: Research Funding; Genentech: Consultancy, Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Mustang Bio: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Regeneron: Consultancy; ADC therapeutics: Consultancy. Till:Mustang Bio: Consultancy, Patents & Royalties, Research Funding; BMS/Juno Therapeutics: Research Funding; Proteios Technology: Consultancy, Current holder of stock options in a privately-held company. Smith:ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (spouse), De Novo Biopharma, Enterome, Genentech, Inc., Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck Sharp and Dohme Corp., MorphoSys, Nanjing Pharmaceu: Research Funding; ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, KITE pharma, Incyte, Numab Therapeutics AG, Abbvie, Coherus Biosciences, advisory board (spouse) Genentech, Inc.: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees. Ujjani:Atara: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy; Pharmacyclics: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Beigene: Consultancy, Honoraria; Lilly: Consultancy, Honoraria, Research Funding; Astrazeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Other: Travel expenses , Research Funding; PCYC: Research Funding. Gopal:Compliment Corporation: Current holder of stock options in a privately-held company; Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy; Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal